Company: Clementia Pharmaceuticals Inc.
Symbol: CMTA
Description: They are a clinical stage biopharmaceutical company that is developing disease-modifying treatments for patients suffering from debilitating bone and other diseases with high unmet medical need.
Shares: 7.15 million
Price Range: $13.00-$15.00
Trade Date: 8/2
Underwriter(s): Morgan Stanley, Leerink Partners
Co-Manager: Wedbush PacGrow, BTIG
Investor Access: This deal can be accessed via the two main underwriters and the two co-managers.
Business:
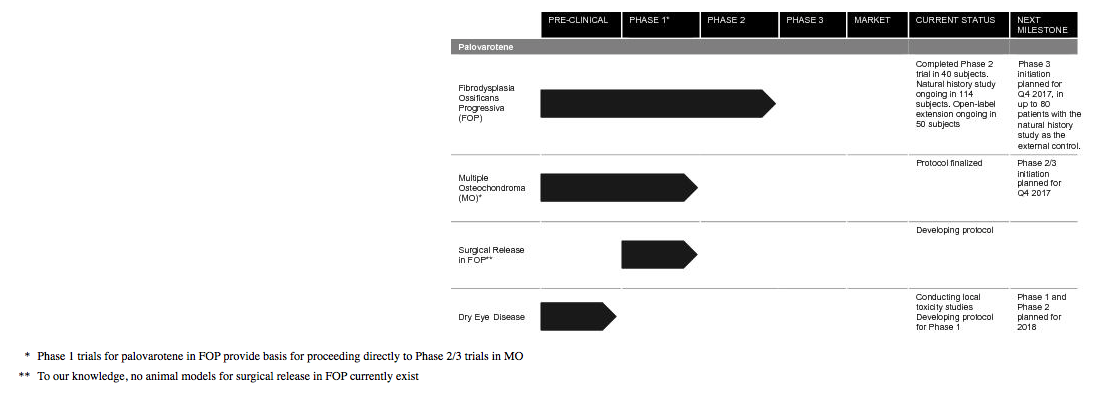
They are developing palovarotene for the treatment of Fibrodysplasia Ossificans Progressiva (FOP) and Multiple Osteochondroma (MO) and have one Phase 3 trial and one Phase 2/3 trial, for two separate indications, planned to commence in 2017. FOP is an ultra-rare, chronic and severely disabling disease of abnormal bone formation, known as heterotopic ossification (HO). Recurrent flare-ups and new bone formation progressively restrict movement by locking joints, leading to cumulative loss of function, disability and early death due to reduced respiratory function. They believe that RARg agonists, such as palovarotene, have the potential for therapeutic use in a broad range of conditions, including diseases like FOP and MO that involve pathological bone formation as well as other indications characterized by excessive fibrosis or scarring such as dry eye disease.
Target Audience:
They estimate that the prevalence of FOP is approximately 1.3 individuals per million lives, or approximately 9,000 globally. As of October 2016, there were known to be 800 diagnosed FOP patients worldwide. There are currently no approved medical treatment options to prevent the formation of heterotopic bone in FOP. In July 2014, the FDA granted Orphan Drug Designation for palovarotene as a treatment for FOP and in November 2014, they received Fast Track Designation. In November 2014, they were granted orphan drug status in the EU. In Japan, they briefed the PMDA on their palovarotene FOP program in January 2017. Also, in July 2017 they received Breakthrough Therapy Designation from the FDA for the prevention of HO in patients with FOP.
They believe that palovarotene treatment could potentially reduce morbidity and deformity and preserve function in patients with MO. . MO affects approximately 20 individuals per million lives or approximately 150,000 globally, which is approximately 15 times greater than FOP.
Their work in FOP, MO, and ocular disorders, as well as in non-clinical studies designed to elucidate RARg agonist biology in a variety of cell systems has provided them with unique insights into the biological effects of systemically administered RARg agonists and their potential therapeutic applications. They believe that RARg selective agonists have substantial untapped therapeutic potential because of their potential anti-fibrotic and tissue regeneration and repair activities and because of their predicted safety profile (if palovarotene is representative of the group).
Book-Building Status: The way the book comes together during the week of the roadshow is the most critical indicator to first-day and first-week performance. IPO Boutique uses its 45 years of experience and sources all over the street to gather daily subscription levels, specific price guidance and what type of investors are currently in the book or are anchoring orders in the book. In addition, recent underwriter performance on sector specific deals is a strong factor that IPO Boutique takes into account when determining if our clients should indicate for any offering.
IPO Boutique subscription clients receive daily updates on this critical information.
Conclusion: IPO Boutique provides ratings, daily commentary and a forecast for how this IPO will open vs. its offering price. We have kept a track record with our performance for last 12 years at our website. Additionally, we have our latest performance results with commentary from the month of May by clicking here.
Indicate with confidence, SUBSCRIBE today.